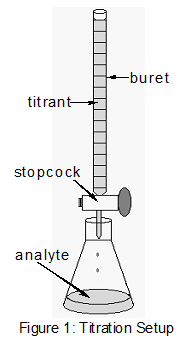
It is type of titration used when back titration or direct titration are insufficient.
Easly It depends on the ability of metal to bind to titrant (e.g: EDTA) more than another metal.
to be clear we will take an example:
principles:
- 1- Ca+2 has higher affinity to bind to EDTA more than Mg+2
- Mg+2 has higher affinity to bind to EBT more than Ca+2
- color of EBT when binds to metal is wine red and has blue color in free form
procedure:
- buret : contain EDTA
- Conical : contain the EBT-Mg+2 and Ca+2
here the analyte (substance wanted to be mesured) is Ca+2, EBT is the indicator and it binds the Mg+2 giving color (wine red). This because Mg+2 has higher affinity to bind to EBT than Ca+2.
Now lets start titration:
- the amount of EDTA added to conical will bind to Ca+2 because its higher affinity to EDTA
- now after adding more EDTA all free Ca+2 will be bound to EDTA
- at the end addition of more EDTA will relase the Mg+2 from EBT and bind to EDTA, so after that free EBT will be in the conical giving blue color which called endpoint of the reaction
so here in replacement titration we depends on affinity of the analyte to both titrant and indicator
for example if we used Murexide indicator the previous reaction can not be occured, because Ca+2 can bind to Murexide.
for example if we used Murexide indicator the previous reaction can not be occured, because Ca+2 can bind to Murexide.
so in replacement titration the analyte has low ability to bind to the indicator or give no sharp endpoint. also you need to use metal has higher affinity to the indicator as shown up.


ليست هناك تعليقات:
إرسال تعليق