Water contain many minerals but, upon boiling there are some minerals called temporary hardness are precipitated. the most mineral precipitated is calcium in the form of calcium carbonate (CaCO3).
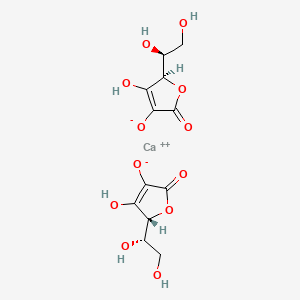
When we add Vitamin.C on water it binds to calcium so upon boiling CaCO3 formation is prevented.So soluble Vitamin.C with calcium still in water.so addition of Vitamin.C prevent calcium loss from boiled water.
If you want to wash your kettle from calcium carbonate precipitation. you can use ascorbic acid or vinegar (acetic acid) or lemonade (citric acid) in washing, because all these acids binds to calcium from calcium carbonate breaking it.


ليست هناك تعليقات:
إرسال تعليق